tudor domain sequence | drosophila tudor website tudor domain sequence The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene . This page will introduce you various decks to defeat Jesse Anderson Lvl 40 to get a high score! Use decks below to farm exclusive rewards cards and to complete event missions! Jesse is not that hard to defeat/farm, but note that you can use only characters in GX world to fight against him.
0 · tudor protein structure
1 · tudor protein
2 · drosophila tudor website
3 · drosophila tudor protein
Gondola Rides at the Venetian: Fake fun in fake Venice - See 2,329 traveller reviews, 1,011 candid photos, and great deals for Las Vegas, NV, at Tripadvisor.
Tudor domains are small 50 amino acid long domains consisting mostly of β-strands [8–11]. The function of tudor domains is not well-established. They are present in a variery of proteins, . It encodes a large protein, Tudor (Tud), of 2 515 amino acids, containing 11 copies of a ∼ 60-residue sequence motif, termed the tudor domain. Tudor domains are best .
Multiple sequence alignment of representative sequences of domains from the Tudor domain ‘Royal family’ depicted using CHROMA (http://www.lg.ndirect.co.uk/chroma/) . The Tudor domain core contains a conserved β-barrel structure, with an aromatic cage for methyl-ligand recognition. Crystal structures of ligand-bound extended Tudor domains .The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene . Tudor domain proteins function as molecular adaptors, binding methylated arginine or lysine residues on their substrates to promote physical interactions and the assembly of .
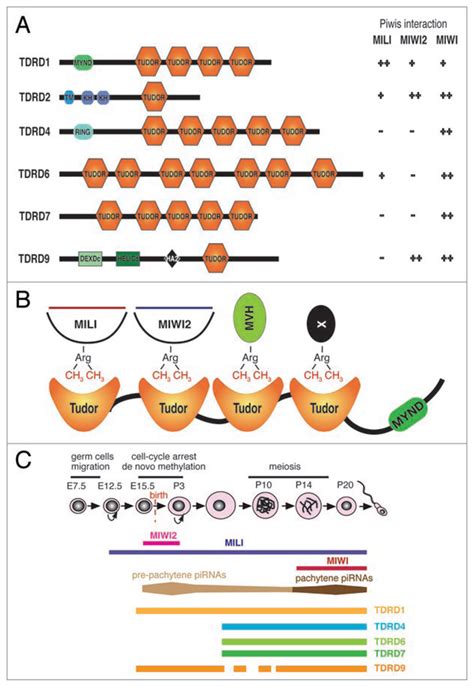
The Tudor Domain. (A) Amino acid sequence of the Tudor domain in SMN (Homo sapiens) in comparison to the 11 Tudor domains of TUD. Hydrophobic residues are highlighted .
Sequence searches (Table 1) show that this new domain is a distant relative of the Tudor domain, named from the Drosophila Tudor protein in which 11 copies are found [13]. Consequently, we . To search for proteins belonging to Agenet/Tudor domain family in plants, we used an Agenet/Tudor sequence from the gene At1g09320 to perform TBLASTN query against .
Tudor Domains as Methyl-Lysine and Methyl-Arginine Readers. M.V. Botuyan, G. Mer, in Chromatin Signaling and Diseases, 2016 Single Tudor Domains of PHF1 and PHF19. The proteins PHF1 and PHF19 (plant homeodomain finger 1 and 19) of the Polycomb-like complex in mammals each harbor a single Tudor domain.The Polycomb-like complex is involved in the .
Sequence searches show that this new domain is a distant relative of the Tudor domain, named from the Drosophila Tudor protein in which 11 copies are found . Consequently, we refer to these Tudor homologs as plant ‘Agenet’ domains, .
Arrows indicate a potential evolutionary track of these Tudor domain classes. (B) Multiple alignments of germline Tudor domain sequence flanking the canonical Tudor core domains, showing 50 amino acids N-terminal to the Tudor domain core. Secondary structure features (green letters: β-strands, blue letters: α-helix) were predicted by NetSurfP . The obtained set was analysed for the presence of TUDOR‐clan similarities as described in the following: Each protein sequence was analysed for its presence in a Pfam24 TUDOR‐clan domain definition with the aim to identify known and .
To identify critical Tudor domains required by leukemia, we evaluated the NCBI Conserved Domains Database and summarized 59 Tudor domains in the mammalian genome (span across 36 proteins; data S1) and developed a custom CRISPR library targeting these Tudor domains with 992 sgRNAs (Fig. 1A; ~16.8 sgRNAs per Tudor domain; fig. S1 and data S2).We . Arrows indicate a potential evolutionary track of these Tudor domain classes. (B) Multiple alignments of germline Tudor domain sequence flanking the canonical Tudor core domains, showing 50 amino acids N-terminal to the Tudor domain core. Secondary structure features (green letters: β-strands, blue letters: α-helix) were predicted by NetSurfP .
Tudor domain-containing (TDRD) proteins, the germline enriched protein family, play essential roles in the process of gametogenesis and genome stability through their interaction with the PIWI-interacting RNA (piRNA) pathway. . Multiple sequence alignment of Tudor domains was done using Clustal W by MEGA7.0 (Kumar et al. 2016) and the . Dear Editor, Drosophila tudor is a maternal effect gene required for germ cell formation and abdominal segmentation during oogenesis 1,2.It encodes a large protein, Tudor (Tud), of 2 515 amino acids, containing 11 copies of a .
Tudor domain as ‘readers’ of histone PTMs. The Tudor domain was named after the Drosophila tudor (tud) gene identified in a screen for maternal-effect recessive lethality or sterility []. Drosophila tud contains eleven repeats of a conserved motif, subsequently termed Tudor, which appears in many proteins throughout various species [19, 20].The Tudor domain typically .tions of the Agenet domain are unknown. Sequence searches (Table 1 ) show that this new domain is a Corresponding author: Chris P. Ponting ([email protected]). distant relative of the Tudor domain, named from the . region of the SMN Tudor domain is structurally coincident with the methylated histone H3-binding region of the HP1 .Sequence searches show that this new domain is a distant relative of the Tudor domain, named from the Drosophila Tudor protein in which 11 copies are found . Consequently, we refer to these Tudor homologs as plant ‘Agenet’ domains, from the Plantagenet dynasty of English monarchs.
The human Polycomb-like protein PHF1 has been implicated in transcription-regulatory and DNA damage repair pathways. A new study demonstrates that the Tudor domain of PHF1 binds histone H3K36me3 .
The tudor domain is composed of four antiparallel β-strands forming a barrel-like structure with an aromatic cage/binding pocket that can bind either methylated arginine or lysine residues [23]. . Structure of PHF20 single Tudor domain 2 fused at its C-terminus to a p53K C 370me2 sequence. (B) Structure of PHF1 single Tudor domain in complex .the tudor domain; 2) the presence of an additional short helix, αC’, in the OB-fold domain of Tud9; and 3) an . modules based on sequence alignment and secondary structure analyses. The .
A Tudor domain is a protein region approximately 60 amino acids in length, which folds into an SH3-like structure with a five-stranded antiparallel beta-barrel form. [1] Tudor domains can further be organized into functional units consisting of either a single Tudor domain, tandem Tudor domains, or hybrid Tudor domains consisting of two Tudor domains linked by an anti-parallel .Tudor domains are small 50 amino acid long domains consisting mostly of β-strands [8–11]. The function of tudor domains is not well-established. They are present in a variery of proteins, including the survival motor neuron (SMN) protein, which .
It encodes a large protein, Tudor (Tud), of 2 515 amino acids, containing 11 copies of a ∼ 60-residue sequence motif, termed the tudor domain. Tudor domains are best characterized by their. Multiple sequence alignment of representative sequences of domains from the Tudor domain ‘Royal family’ depicted using CHROMA (http://www.lg.ndirect.co.uk/chroma/) and a 75% consensus threshold. The Tudor domain core contains a conserved β-barrel structure, with an aromatic cage for methyl-ligand recognition. Crystal structures of ligand-bound extended Tudor domains (eTuds) have.
The Tudor domain is a methyl-lysine and methyl-arginine binding domain present in proteins involved in cellular functions as diverse as DNA transcription, RNA metabolism, gene silencing, the transmission of epigenetic posttranslational modifications, and the maintenance of . Tudor domain proteins function as molecular adaptors, binding methylated arginine or lysine residues on their substrates to promote physical interactions and the assembly of macromolecular complexes. The Tudor Domain. (A) Amino acid sequence of the Tudor domain in SMN (Homo sapiens) in comparison to the 11 Tudor domains of TUD. Hydrophobic residues are highlighted in blue and negatively.
Sequence searches (Table 1) show that this new domain is a distant relative of the Tudor domain, named from the Drosophila Tudor protein in which 11 copies are found [13]. Consequently, we refer to these Tudor homologs as plant ‘Agenet’ domains, from the .
tudor protein structure
tudor protein
Go to FashionReps r/FashionReps • by Mean-Substance-2937. lv belts. comments sorted by Best Top New Controversial Q&A Add a Comment. Mean-Substance-2937 • .
tudor domain sequence|drosophila tudor website